Mmeghachi omume nke ogige mercury
Metallic Mercury na ogige ya na-egbu egbu maka ihe ndị dị ndụ. Nke a bụ eziokwu karịsịa maka ogige ndị na-agbaze nke ukwuu na mmiri. A ghaghị ịkpachara anya mgbe ị na-anwale nchikota nke ihe a pụrụ iche (mercury bụ naanị ígwè nke bụ mmiri mmiri na ụlọ okpomọkụ). Nrube isi n'iwu ndị bụ isi nke kemist? ga-enye gị ohere ịme ọtụtụ nnwale na ogige mercury n'enweghị nsogbu.
Na nnwale nke mbụ, anyị na-enweta aluminom amalgam (ihe ngwọta nke ígwè a na mmiri mmiri mercury). Mercury (II) ngwọta Hg nitrate (V) Hg (NO3)2 na mpempe aluminom waya (foto 1). A na-etinye mkpanaka aluminom (nke ọma nke ọma nke nkwụnye ego) na tube ule na ngwọta nke nnu mercury soluble (foto 2). Mgbe oge ụfọdụ gasịrị, anyị nwere ike ịhụ ntọhapụ nke gas na-egosipụta n'elu waya (foto 3 na 4). Mgbe o wepụsịrị mkpanaka ahụ site na ngwọta, ọ na-apụta na a na-ekpuchi ụrọ ahụ na mkpuchi na-egbuke egbuke, na mgbakwunye, anyị na-ahụkwa bọọlụ nke mercury dara (foto 5 na 6).
Chemistry - ahụmahụ nke ijikọta mercury
N'okpuru ọnọdụ nkịtị, a na-ekpuchi elu aluminom na oyi akwa dabara adaba nke aluminom oxide.2O3na-ekewapụ ígwè ahụ nke ọma site na mmetụta gburugburu ebe obibi ike ike. Mgbe ihichachara na imikpu mkpanaka ahụ na ngwọta nke nnu mercury, a na-ewepụ Hg ion2+ aluminom na-arụ ọrụ karịa
Mercury nke etinyere n'elu mkpanaka ahụ na-emepụta amalgam na aluminum, nke na-eme ka ọ na-esiri oxide ike ịrapara na ya. Aluminom bụ ígwè na-arụsi ọrụ ike (ọ na-emeghachi na mmiri iji hapụ hydrogen - a na-ahụ ihe ọkụkụ gas), na iji ya mee ihe dị ka ihe nhazi ga-ekwe omume n'ihi mkpuchi mkpuchi oxide.
Na nnwale nke abụọ, anyị ga-achọpụta ion ammonium NH.4+ iji Nessler's reagent (onye German chemist Julius Nessler bụ onye mbụ ji ya mee nyocha na 1856).
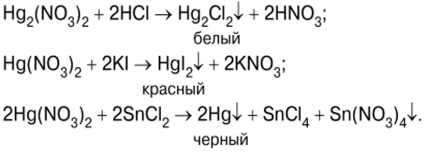
Nlele na mmeghachi omume nke hops na ogige mercury
Nnwale ahụ na-amalite site na mmiri ozuzo nke mercury(II) iodide HgI.2, mgbe agwakọta ngwọta nke potassium iodide KI na mercury (II) nitrate (V) Hg (NO)3)2 (Foto nke 7):
Oroma-acha uhie uhie nke HgI2 (foto 8) wee mesoo ya na oke potassium iodide ngwọta iji nweta ngwakọta mgbagwoju anya nke usoro K.2HgI4 ? Potassium tetraiodercurate (II) (Foto 9), nke bụ Nessler reagent:
Site na ihe na-esi na ya pụta, anyị nwere ike ịchọpụta ion ammonium. Ngwọta nke sodium hydroxide NaOH na ammonium chloride NH ka ga-achọrọ.4Cl (Foto nke 10). Mgbe agbakwunyere ntakịrị ihe ngwọta nnu ammonium na Nessler reagent na alkalizing na ọkara na isi siri ike, anyị na-ahụ nhazi nke agba odo-oroma nke ọdịnaya tube ule. Enwere ike dee mmeghachi omume ugbu a dịka:
Ngwakọta mercury nke sitere na ya nwere usoro dị mgbagwoju anya:
A na-eji ule Nessler na-enwe mmetụta nke ukwuu iji chọpụta ọbụna akara nke nnu ammonium ma ọ bụ amonia n'ime mmiri (dịka mmiri mgbata).

